Labs & Specimens
The Lab & Specimen Regulation Schedule 2 - Requisition by midwife Section 18 and subsection 25.1 for midwives related to point of care testing were updated on December 19, 2025. This revised regulation includes an additional 34 labs, specimens and point of care tests (POCTs) that midwives in Ontario may now order on their own authority. This page provides an overview of each of the new tests added. POCTs can be found at the bottom of the page. By opening the drop down menu for a test below you will find:
BACKGROUND
- A high level overview of what is being tested and a brief review of relevant physiology.
COMMON INDICATIONS
- Some common indications to order this test. This is not an exhaustive list of indications. Midwives should order testing based on community standards, policies and protocols, and use their knowledge and judgment in the judicious and appropriate ordering of testing.
SAMPLE COLLECTION
- Type of sample (e.g., blood/serum, urine) and collection method (e.g., type of vacutainer tube). Midwives should defer to their local laboratories for any preferred collection methods identified in that community.
reference values
- Where applicable, the normal reference range is provided. These values may differ from those used by various community and/or hospital laboratories. Midwives should refer to the specific reference ranges implemented in their community.
SOURCES
- Additional resources (e.g., AOM webinars) and relevant literature.
Alanine transaminase (ALT)
BACKGROUND
Also known as alanine aminotransferase (both terms shortened to ALT), this enzyme is highly concentrated in the liver but is also found in low concentrations in skeletal muscle and kidneys. An increase in ALT serum levels is the most specific marker of liver cell damage.
COMMON INDICATIONS
- Recommended laboratory test for ICP investigation (SOGC ICP guideline), and preeclampsia screening (SOGC HDP guideline)
SAMPLE COLLECTION
- Serum sample: gold top or red top (SST) vacutainer tube, or light green lithium-heparin tube.
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| U/L | 7 - 41 | 3 – 30 | 2 – 33 | 2 – 25 |
| µkat/L | 0.12 - 0.68 | 0.05 – 0.5 | 0.03 – 0.55 | 0.03 – 0.42 |
SOURCES
- Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005 Feb 1;172(3):367-79.
- Reference values: Alanine aminotransferase, ALT, SGPT (serum). Perinatology.com. 2010. https://www.perinatology.com/Reference/Reference%20Ranges/ALT,SGPT.htm
Alloimmunized fetal blood group genotyping
BACKGROUND
Alloimmunized fetal blood group genotyping detects fetal blood group antigens RhD, C, c, D and/or K (Kell) to identify pregnancies at risk of hemolytic disease of the fetus and newborn (HDFN) requiring further monitoring while allowing those carrying antigen negative fetuses to transition to routine care.
For more information, visit Prenatal Screening Ontario
COMMON INDICATIONS
- Testing for pregnant individuals with antibodies D, C, c, E, and/or K (Kell)
SOURCES
For additional information, watch this AOM webinar: Non-invasive fetal blood group genotyping: Moving towards precision care for pregnant individuals in Ontario (June 2025)
Prenatal Screening Ontario: www.prenatalscreeningontario.ca
Bile acid total and fractionation
Background
Bile acids are cytotoxic steroids produced in the liver and then secreted into bile and stored in the gallbladder. Bile acids have several functions, including a role in digestion of fat. Fetal production of bile acids begins around 12 weeks. Bile acids can cross the placenta. In a typical pregnancy, there is a fetal to maternal gradient facilitating clearance of bile acids, which protects the fetus from cytotoxicity. When bile acids are significantly increased in the pregnant person, as with intrahepatic cholestasis of pregnancy (ICP), this protective gradient is reversed, and bile acids accumulate on the fetal side.
COMMON INDICATIONS
- Included in recommended tests for ICP (SOGC guideline No. 452)
SAMPLE COLLECTION
- Serum sample: red top vacutainer tube. Do not use separator gel. Store and ship refrigerated.
- Collection notes: fasting not recommended for ICP evaluation; increased sensitivity in non-fasting samples for ICP investigation.
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| µmol/L | 0.3 - 10 | 0 - 4.9 | 0 - 9.1 | 0 - 11.3 |
- SOGC states that while normal bile acid ranges vary by laboratory, the upper limit is generally considered 10 µmol/L for a non-pregnant adult. The SOGC endorses a value of non-fasting TSBA > 19 µmol/L as a defining feature of ICP. (Hobson, 2024)
SOURCES
- Chen I, Cassaro S. Physiology, bile acids. Stat Pearls 2023 May 1. StatPearls Publishing.
- Hobson SR, Cohen ER, Gandhi S, Jain V, Niles KM, Roy-Lacroix MÈ, Wo BL. Guideline No. 452: diagnosis and management of intrahepatic cholestasis of pregnancy. Journal of Obstetrics and Gynaecology Canada. 2024 Aug 1;46(8):102618.
- AOM Webinar: "SOGC Guideline Update: Intrahepatic Cholestasis in 2025"
Bilirubin - unconjugated
Neonatal
BACKGROUND
Almost all hyperbilirubinemia in the immediate newborn period is unconjugated. Most neonatal bilirubin is produced by the breakdown of hemoglobin into unconjugated bilirubin, which then binds to albumin in the blood and is transported to the liver. Once in the liver, it is conjugated to make it water soluble, and then it is excreted in the bile.
COMMON INDICATIONS
- Screening and follow up testing for neonatal hyperbilirubinemia
- For guidance on the detection and management of newborn hyperbilirubinemia, consult the Canadian Paediatric Society Guidelines (updated March 2025)
SAMPLE COLLECTION
- Included in total serum bilirubin (TSB) assessments: serum sample (capillary/heelstick) in gold (or green) microtainer
- Protect sample from light
reference values
- Consult a neonatal hyperbilirubinemia nomogram to determine if a value is normal or requires follow up and/or treatment in consideration of variables such as risk factors and hours of age.
SOURCES
- Dysart KC, Pekarsky AR. Neonatal hyperbilirubinemia. Merck Manual. Jun 2025. Available from: https://www.merckmanuals.com/professional/pediatrics/metabolic-electrolyte-and-toxic-disorders-in-neonates/neonatal-hyperbilirubinemia
- Ng E, et al. Guidelines for detection and management of hyperbilirubinemia in term and late preterm newborns (≥35 weeks gestational age). Canadian Paediatric Society. March 2018. Available from: https://cps.ca/en/documents/position/hyperbilirubinemia-newborns.
- https://sunnybrook.ca/content/?page=labratory-medicine-neonatal-specimen-collection
- AOM resources for hyperbilirubinemia published January 2026: CPG #18: Management of Hyperbilirubinemia in Healthy Term and Late Preterm Neonates, CPG Summary: Hyperbilirubinemia, and Clinical Pathway Manual for Midwifery Hyperbilirubinemia Screening and Management of Phototherapy
Chlamydia by Nucleic Acid Amplification Test (NAAT)
BACKGROUND
Nucleic acid amplification testing (NAAT) is a PCR assay for the qualitative detection of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG). NAAT is the recommended method for initial screening or testing for CT. Simultaneous testing of both CT and NG occurs from a single specimen (i.e., one collection kit). Testing for either CT or NG as a single test without the other by NAAT is not available, and requests will only be tested with the CT/NG duplex assay.
For more details about NAAT for Chlamydia trachomatis, including lab acceptance/rejection criteria, specimen collection and handling criteria, turnaround time and interpretation, visit the Public Health Ontario website.
COMMON INDICATIONS
- Routine screening in pregnancy, test of cure (NB: NAAT is only for duplex assay of CT and NG so may have limited applicability for TOC)
SAMPLE COLLECTION
- Vaginal swab is "specimen of choice" per Public Health Ontario, but can be performed on first-void urine samples (transferred to a Roche cobas® PCR Urine Sample Kit using the disposable pipette, pictured below)
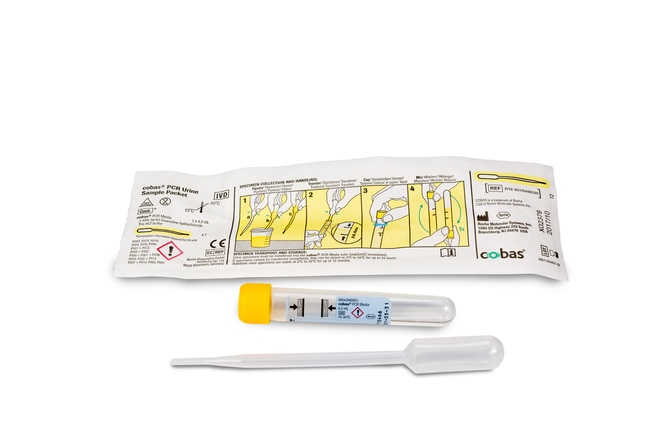
SOURCES
- https://www.publichealthontario.ca/en/Laboratory-Services/Test-Information-Index/Chlamydia-trachomatis-NAAT-Swabs
- https://diagnostics.roche.com/content/dam/diagnostics/us/en/products/c/cobas-liat-support/cobas-pcr-urine-sample-kit-ifu.pdf
Creatinine, including estimated glomerular filtration rate (eGFR)
BACKGROUND
Glomerular filtration rate (GFR) describes the flow rate of filtered fluid through the kidneys. The gold-standard test involves inulin injection and clearance measurement – an invasive, time consuming, and more expensive test. An alternative test looks at the estimated GFR (eGFR) based on creatinine levels. Creatinine is a waste product excreted by the kidneys, and a biochemical marker of renal function. It can be detected in urine or blood. Due to physiologic glomerular hyperfiltration in pregnancy, eGFR results should be interpreted as part of a larger clinical picture.
COMMON INDICATIONS
- HDP screening including preeclampsia screening
- While serum samples more commonly used, creatinine test may also refer to urine protein:creatinine ratio as part of HDP testing (NB: both protein and creatinine are listed in the Midwifery lab regulation)
SAMPLE COLLECTION
- Serum sample in gold top SST, red top or green top
reference values
Creatinine
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| mg/dL | 0.5 - 0.9 | 0.4 - 0.7 | 0.4 - 0.8 | 0.4 - 0.9 |
| µmol/L | 44 - 90 (mean 60) | 35 - 62 | 35 - 71 | 35 - 80 |
- Serum creatinine values > 76 μmol/l (0.86 mg/dl) in the 1st trimester, 72 μmol/l (0.81 mg/dl) in the 2nd trimester, and 77 μmol/l (0.87 mg/dl) in 3rd trimester are be considered to be outside the upper limit of normal for pregnancy. (Wiles 2018)
eGFR
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| mL/min | 106 - 132 | 131 - 166 | 135 - 170 | 117 - 182 |
equity considerations
- For many years, the equation used to calculate kidney function included adjustments for Black race resulting in an upward ‘correction’ in eGFR results. (Parekh 2022) The inclusion of race in kidney function estimates is an example of race correction, and is inherently harmful. Race is not a biological factor, it is a social construct. So, when health systems (including laboratories) treat race as a biological variable, their work is based on false assumptions, and it produces false results. In Canada, Black people are referred later for kidney care. The statistical bias in equations including Black race as a factor leads to an overestimation of kidney function. This is an important factor that may prevent timely kidney care. (Parekh 2022)
- Updated equations that omit race but include other factors were developed in 2021 and found to be more accurate. The evidence supports the elimination of race correction to reduce inequities in access to timely care. (Parekh 2022) In Ontario, the process of removing race from eGFR calculations is underway (Brimble 2021), and most major labs (e.g., Dynacare, Life Labs) have updated to the new calculation which does not include race. Consider verifying with your local community and hospital-based laboratories whether they have updated to the race-free “CKD-EPI 2021” equation for eGFR.
SOURCES
- Brimble KS, Treleaven D, Cooper R, Blake PG. Removal of eGFR adjustment for race in Ontario. 2021 Apr 21. https://www.cmaj.ca/content/removal-egfr-adjustment-race-ontario
- Harel Z, McArthur E, Hladunewich M, Dirk JS, Wald R, Garg AX, Ray JG. Serum creatinine levels before, during, and after pregnancy. Jama. 2019 Jan 15;321(2):205-7.
- Parekh RS, Perl J, Auguste B, Sood MM. Elimination of race in estimates of kidney function to provide unbiased clinical management in Canada. CMAJ. 2022 Mar 21;194(11):E421-3.
- Wiles K, Bramham K, Seed PT, Nelson-Piercy C, Lightstone L, Chappell LC. Serum creatinine in pregnancy: a systematic review. Kidney international reports. 2018 Oct 29;4(3):408-19.
- https://www.endracecorrection.com/what-is-race-correction/
- https://www.perinatology.com/Reference/Reference%20Ranges/Creatinine.htm
- https://www.perinatology.com/Reference/Reference%20Ranges/GFR.htm
Cytomegalovirus (CMV) by PCR
BACKGROUND
Cytomegalovirus (CMV) is a common virus and for most healthy people who have had it, they would not have had any specific signs or symptoms of exposure. CMV infection may present as fatigue, sore throat, fever and swollen glands. Infection during pregnancy may result in congenital cytomegalovirus (cCMV) which can impact fetal growth and the development of the brain, inner ear and eye. During pregnancy, IUGR, oligohydramnios and abnormal fetal findings may present with CMV infection. There are no CMV vaccines or treatment for CMV in pregnancy to reduce the risk of cCMV. Primary infection during pregnancy carries a risk of cCMV of 30% to 40%, so client education and prevention are important.
COMMON INDICATIONS
- Screening for those with risk factors for CMV exposure, or testing if fetal ultrasound findings suggestive of cCMV, symptoms consistent with CMV (generalized illness, mononucleosis-life syndrome) and undifferentiated hepatitis.
SAMPLE COLLECTION
From Public Health Ontario: “When CMV infection is suspected in a pregnant [person]... order CMV serology... on the PHO requisition form, be sure to request both CMV IgM and IgG. Include all relevant clinical information (e.g. pregnancy, suspected congenital infection, etc.), onset date and signs and symptoms.”
- Part of the TORCH test
- Serum (pregnant person): gold top vacutainer (SST) or red top vacutainer
- Newborn Screening Ontario: Beginning March 4, 2024, all babies for whom consent is provided to participate in hearing screening through the Infant Hearing Program (IHP) will receive hearing loss risk factor screening by NSO. Babies who screen positive for CMV or genetic risk factors for PHL will be contacted by Newborn Screening Ontario to discuss the results and recommended follow-up. The primary care provider will also be informed in order to follow up.
SOURCES
- Boucoiran I, Yudin M, Poliquin V, Caddy S, Gantt S, Castillo E. Guideline no. 420: cytomegalovirus infection in pregnancy. Journal of Obstetrics and Gynaecology Canada. 2021 Jul 1;43(7):893-908.
- Newborn Screening Ontario. Congenital Cytomegalovirus. https://www.newbornscreening.on.ca/en/screening/types-of-screening/permanent-hearing-loss/congenital-cytomegalovirus
- https://www.publichealthontario.ca/en/Laboratory-Services/Test-Information-Index/Cytomegalovirus-Serology
Fetal fibronectin
BACKGROUND
“Fetal Fibronectin (fFN) is a glycoprotein produced by the chorionic membranes and is localized to the deciduas basalis adjacent to the intervillous space. Its primary purpose appears to be that of an adhesion molecule (tissue glue) which helps bind the chorionic membranes to the underlying maternal decidua.
It is normally found in cervico-vaginal secretions until 22 weeks gestation but is virtually never found between 24 and 34 weeks gestation unless the cervix has undergone premature effacement and dilatation, usually in association with symptomatic uterine contractions. It can also be released in response to inflammation or separation of amniotic membranes from the deciduas. There is a strong association between the presence of Fetal Fibronectin in cervicovaginal secretions and preterm labour after 24 weeks gestation.” (MOH 2008)
Fetal fibronectin testing can help determine if there is an increased risk for preterm birth in pregnant people at 24-34 weeks gestational age presenting with signs of preterm labour, and guide decision making around admission/transfer to an appropriate facility.
COMMON INDICATIONS
- Suspected preterm labour at 24-34 completed weeks of pregnancy
SAMPLE COLLECTION
- fFn must be completed PRIOR to any other examinations (e.g., digital vaginal exam for cervical assessment, transvaginal ultrasound) or testing (e.g., amniotic swab). These can alter the accuracy of the test (e.g., increase false positives).
- fFn should be collected during a sterile speculum exam. The use of lubricant on the speculum can alter the accuracy of the test (e.g., increase false negatives).
- If any examinations, tests, or lubricants have been conducted prior to fFn, wait 24 hours before collecting the fFn sample.
- Contraindications for processing fFn: GA < 24 weeks or > 34 completed weeks, PROM, cervix ≥ 3cm dilation, cervical cerclage, active vaginal bleeding, vaginal exam or sexual intercourse in the past 24 hours.
reference values
The test result is reported as a qualitative positive or negative value rather that a quantitative value.
- Negative: indicates that delivery is not likely to take place within 7-14 days (>95% accuracy)
- Positive: indicates a higher risk of preterm delivery (16 – 17%)
SOURCES
- Ontario Ministry of Health. Fetal fibronectin: Guideline for the use in the management of preterm labour. 2008. Available from: https://www.pcmch.on.ca/wp-content/uploads/2022/02/fetalfibronectin_guidelines.pdf
- https://www.pcmch.on.ca/fetal-fibronectin-testing/
- Berghella V, Saccone G. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database of Systematic Reviews. 2019(7).
Fetal RhD
BACKGROUND
Fetal RHD screening for RhD negative pregnant individuals is a non-invasive test that looks at cell-free DNA in the pregnant person’s blood to determine the fetus’ RhD blood type. In Ontario, this test will soon be publicly funded for those with OHIP, with a launch anticipated in 2026 . Determining the fetal RhD blood type prenatally can guide the need for RhIG, minimizing unnecessary use of this human blood product and promoting it's conservation.
For more information, visit Prenatal Screening Ontario
COMMON INDICATIONS
- Prenatal screening for RhD negative individuals
SOURCES
For additional information, watch this AOM webinar: Non-invasive fetal blood group genotyping: Moving towards precision care for pregnant individuals in Ontario (June 2025)
- Prenatal Screening Ontario: www.prenatalscreeningontario.ca
Gamma-glutamyl transferase (GGT)
BACKGROUND
Gamma-glutamyl transferase (GGT) is a glycoprotein located on the membranes of cells with high secretion/absorption activity. The primary function of GGT is as a catalyst for the transfer of gamma-glutamyl groups to acceptor peptides and amino acids. It is a liver enzyme commonly included in liver function testing and is also used in biliary assessments. GGT has a high sensitivity for liver damage, though a low specificity for aetiology.
COMMON INDICATIONS
- Intrahepatic cholestasis of pregnancy (ICP) testing
SAMPLE COLLECTION
- Serum sample in a gold (SST), red or green top vacutainer
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| U/L | 9 - 58 | 2 - 23 | 4 - 22 | 3 - 26 |
| µkat/L | 0.15 - 0.97 | 0.03 - 0.38 | 0.07 - 0.37 | 0.05 - 0.43 |
-
In the case of intrahepatic cholestasis of pregnancy, GGT levels may be normal or moderately elevated. If the GGT is significantly raised, alternative diagnoses should be considered. (Hobson 2024)
SOURCES
- Hobson SR, Cohen ER, Gandhi S, Jain V, Niles KM, Roy-Lacroix MÈ, Wo BL. Guideline No. 452: diagnosis and management of intrahepatic cholestasis of pregnancy. Journal of Obstetrics and Gynaecology Canada. 2024 Aug 1;46(8):102618.
- Lala V, Zubair M, Minter D. Liver function tests. StatPearls. 2023 Jul 30.
- https://www.perinatology.com/Reference/Reference%20Ranges/GT.htm
Glycosylated hemoglobin—HbA1c
BACKGROUND
Glycosylated, or glycated, hemoglobin (HbA1c) is used to measure glucose control levels. HbA1c indicates average blood sugar levels over the last 90 days (expressed as a percentage). “Hemoglobin becomes glycated or coated with glucose from the bloodstream. As blood glucose levels increase, more glucose attaches to the hemoglobin protein, resulting in a higher A1c value. Since red blood cells have an average lifespan of about 3 months, the A1c test measures hemoglobin levels in the bloodstream over this period, making it a reliable indicator of blood sugar control.” (Eyth 2025)
COMMON INDICATIONS
- Screening for gestational diabetes mellitus (GDM), glycemic control monitoring in pregnancy for those with pre-existing diabetes, postpartum testing following pregnancy with GDM
- NB: The Diabetes Canada Clinical Practice Guideline recommends early screening ( < 20 weeks) with an A1c in people at high risk of undiagnosed type 2 diabetes, to identify diabetes that pre-dates pregnancy. A glucose challenge test is the preferred approach for GDM screening at 24 to 28 weeks.
SAMPLE COLLECTION
- Serum sample, lavender top vacutainer
- For samples sent to Dynacare laboratories, a single lavender tube can be used for CBC and HbA1c for the same client (no longer need to send 2 lavender tubes as of December 2023).
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| % | 4 - 6 | 4 - 6 | 4 - 6 | 4 - 7 |
| Proportion of total Hb | 0.04 - 0.06 | 0.04 - 0.06 | 0.04 - 0.06 | 0.04 - 0.07 |
SOURCES
- https://www.perinatology.com/Reference/Reference%20Ranges/Hemoglobin%20A1C.htm
- Berger H, Gagnon R, Sermer M. Guideline no. 393-diabetes in pregnancy. Journal of Obstetrics and Gynaecology Canada. 2019 Dec 1;41(12):1814-25.
- Eyth E, Zubair M, Naik R. Hemoglobin A1c. InStatPearls. 2025 Jun 2. StatPearls Publishing.
- Feig DS, Berger H, Donovan L, Godbout A, Kader T, Keely E, Sanghera R. Diabetes and pregnancy. Can J Diabetes. 2018;42(3):S255-82.
Gonorrhea by Nucleic Acid Amplification Test (NAAT)
BACKGROUND
Nucleic acid amplification testing (NAAT) is a PCR assay for the qualitative detection of Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT). NAAT is the recommended method for initial screening or testing for NG. Simultaneous testing of both NG and CT occurs from a single specimen (i.e., one collection kit). Testing for either NG or CT as a single test without the other by NAAT is not available, and requests will only be tested with the CT/NG duplex assay.
For more details about NAAT for Neisseria gonorrhoeae, including lab acceptance/rejection criteria, specimen collection and handling criteria, turnaround time and interpretation, visit the Public Health Ontario website.
COMMON INDICATIONS
- Routine screening in pregnancy, test of cure (NB: NAAT is only for duplex assay of CT and NG so may have limited applicability for TOC)
SAMPLE COLLECTION
- Vaginal swab is "specimen of choice" per Public Health Ontario, but can be performed on first-void urine samples (transferred to a Roche cobas® PCR Urine Sample Kit using the disposable pipette, pictured below)

SOURCES
- https://www.publichealthontario.ca/en/Laboratory-Services/Test-Information-Index/Chlamydia-trachomatis-NAAT-Swabs
- https://diagnostics.roche.com/content/dam/diagnostics/us/en/products/c/cobas-liat-support/cobas-pcr-urine-sample-kit-ifu.pdf
Hemoglobin electrophoresis or chromatography to include Hb A2 (Hemoglobin A2) fraction
BACKGROUND
- Electrophoresis is the process of separating charged particles (such as proteins) based on their charge and size using an electrical field.
- Chromatography, such as high-performance liquid chromatography (HPLC), is the process of separating particles (such as proteins) based on different physiochemical properties such as size, charge, hydrophobicity or other specific affinities.
- Testing a serum sample with electrophoresis or chromatography can detect abnormal hemoglobin patterns indicative of various hemoglobinopathies (e.g., B-thalassemia, hemoglobin variants like HbS). Typically in adults, the most prominent type of hemoglobin, HbA, contains 2 alpha chains and 2 beta chains. Hemoglobin electrophoresis and chromatography can detect a wide range of Hb beta chain variants such as HbA2, a variant consisting of 2 alpha chains and 2 delta chains. HbA2 is linked to B-thalassemia (a condition involving a quantitative deficit of beta-globin).
COMMON INDICATIONS
- Screening for hemoglobinopathy
- Should not be repeated if previously screened at any point (should be performed once per lifetime)
- NB: Hb electrophoresis will not reliably identify all states of α-thalassemia, and it does not provide information about zygosity (e.g., cis vs. trans deletions)
SAMPLE COLLECTION
- Serum sample in lavender top vacutainer
SOURCES
- For additional information, watch this AOM webinar: Hemoglobinopathies in pregnancy: Who, why and how to screen (March 2025)
-
Barrett AN, Saminathan R, Choolani M. Thalassaemia screening and confirmation of carriers in parents. Best Practice & Research Clinical Obstetrics & Gynaecology. 2017 Feb 1;39:27-40.
-
Campbell JS. Alpha and beta thalassemia. Am Fam Physician. 2009;80(4):339-44.
Hepatitis B surface antigen (HBsAg)
BACKGROUND
Hepatitis B is a virus that causes acute and chronic liver infection. Without treatment, chronic infection with viral hepatitis can lead to cirrhosis and liver cancer. It is an enveloped DNA virus, with an outer envelope component of hepatitis B surface antigen (HBsAg). An evaluation in Ontario from 2012-2016 found an overall prenatal HBV prevalence rate of 0.63%. In regions with longstanding universal birth dose vaccination, such as in Northern Ontario, prevalence rates were as low as 0.06 – 0.07%, whereas in in areas without birth dose vaccination the rate was from 3-fold to 25-fold higher (such as North Toronto with a 1.5% prevalence rate).
Following a positive HBsAg test in pregnancy, hepatitis B envelope antigen (HBeAg) and HBV DNA (viral load testing) should be completed, which may require referral for access to testing for midwifery clients. An Ontario study from 2012–2016 found that following a HBsAg positive test in pregnancy, only 38% of pregnant individuals are screened for HBV DNA in the pregnancy. This is a gap in identifying pregnant individuals who may be eligible for antiviral treatment for the prevention of vertical transmission and to reduce the risk of vaccine failure in infants.
COMMON INDICATIONS
- Routine prenatal screening for hepatitis B virus (HBV)
SAMPLE COLLECTION
- Serum sample in a serum separator gold top vacutainer (preferred) or red top tube
- If sample is for a combination of additional public health prenatal serology (e.g., HIV, syphilis, rubella) then be sure that the 5mL vacutainer tube is FULL
- Specimens received >6 days post-collection will not be tested
SOURCES
- For additional information, watch this AOM webinar: Leading practice change: Viral hepatitis in pregnancy (Oct 2023)
- Biondi MJ, Marchand-Austin A, Cronin K, Nanwa N, Ravirajan V, Mandel E, Goneau LW, Mazzulli T, Shah H, Capraru C, Janssen HL. Prenatal hepatitis B screening, and hepatitis B burden among children, in Ontario: a descriptive study. Cmaj. 2020 Oct 26;192(43):E1299-305.
- Castillo E, Murphy K, van Schalkwyk J. No. 342-hepatitis B and pregnancy. Journal of Obstetrics and Gynaecology Canada. 2017 Mar 1;39(3):181-90.
- Mendlowitz AB, Feld JJ, Biondi MJ. Hepatitis B and C in pregnancy and children: a Canadian perspective. Viruses. 2022 Dec 29;15(1):91.
- Wilkins T, Sams R, Carpenter M. Hepatitis B: screening, prevention, diagnosis, and treatment. American family physician. 2019 Mar 1;99(5):314-23.
Lactate dehydrogenase (LDH), total
BACKGROUND
Lactate dehydrogenase (LDH), an enzyme involved in cellular metabolism, is present in almost all tissues of the body and in high concentrations in muscle, liver and kidney tissue. LDH levels are elevated in various conditions including tissue ischemia, hemolysis and in conditions like preeclampsia. Serum LDH level can be used as a biomarker for cellular damage consistent with the placental, renal and hepatic effects of preeclampsia. There is a correlation between LDH levels and the severity of preeclampsia.
COMMON INDICATIONS
- Testing and monitoring for preeclampsia
SAMPLE COLLECTION
- Serum sample in a gold, red or green top vacutainer
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| (I)U/L | 115 - 211 | 78 - 433 | 80 - 447 | 82 - 524 |
| µkat/L | 1.9 - 3.5 | 1.3 - 7.2 | 1.3 - 7.5 | 1.4 - 8.8 |
- Normal pregnancy can result in elevated LDH compared to non-pregnant values. Some research indicates that LDH levels of 600 IU/L are commonly observed in normal pregnancies, while levels exceeding this threshold (>600 IU/L) have been associated with conditions such as preeclampsia and eclampsia.
- In one study, LDH levels exceeding 800 IU/L were observed in 26% of people with severe preeclampsia
- Research has shown that LDH levels are notably higher in pregnant people with severe preeclampsia compared to those with mild preeclampsia. Additional studies found a significant association between high LDH levels and adverse outcomes in pregnancies impacted by preeclampsia, such as perinatal mortality and neonatal complications.
SOURCES
- Deeksha HS, Pajai S, Eleti MR, Navalihiremath VU. A comprehensive review on serum Lactate Dehydrogenase (LDH) and uric acid in preeclampsia: implications for maternal health and disease severity. Cureus. 2024 Mar 25;16(3).
- Farhana A, Lappin SL. Biochemistry, lactate dehydrogenase. InStatPearls [internet] 2023 May 1. StatPearls Publishing.
- https://www.perinatology.com/Reference/Reference%20Ranges/LDH.htm
Non-invasive prenatal testing (NIPT), common aneuploidies
BACKGROUND
Non-invasive prenatal testing (NIPT) is a prenatal genetic screening test that can be conducted after 9-10 weeks gestational age. This test is offered to pregnant people either as a publicly-funded or private-pay test, depending on the client’s circumstances. NIPT is a blood test that does not routinely involve an ultrasound. If a client chooses to have NIPT, then other genetic screening tests such as enhanced first trimester screening (eFTS) are not indicated. However, an 11-14 week nuchal translucency ultrasound (without the serum sample component of eFTS) should be offered for additional information not provided by NIPT alone.
For a step-by-step guide to determining eligibility, funding, ordering, interpreting and communicating NIPT results for clients, please see the Prenatal Screening Ontario NIPT Guide.
BORN Ontario has notified laboratory partners Lifelabs and Dynacare about this update. If the midwife is having any issues submitting requisition for NIPT, please reach out to the laboratory directly. For other questions related to ordering or interpreting results, midwives are encouraged to contact PSO’s information line, staffed by genetic counsellors:
- Phone: 613-737-2281/1-833-351-6490 (toll-free)
- Email: pso@bornontario.ca
- NB: The PSO info line will be closed from December 24, 2025 - Jan 2, 2026.
COMMON INDICATIONS
- Prenatal genetic screening
SAMPLE COLLECTION
- Serum sample
- Publicly funded NIPT is available through two provincial labs: LifeLabs or Dynacare.
- Privately-funded NIPT is available through LifeLabs and Dynacare as well as other laboratories outside of Canada (sample can be collected in Ontario but must be sent to these external labs for analysis).
- Life Labs Panorama NIPT: Cell-Free DNA BCT tubes (Streck tubes): Tan colour top
- Dynacare Harmony NIPT: Two tubes in kit (must fill each at least ½ full or greater). Follow instructions in kit.
SOURCES
For additional information, watch this AOM webinar: Non-Invasive Prenatal Testing (NIPT): what Ontario clinicians need to know (Nov 2023)
- https://www.prenatalscreeningontario.ca/
Phosphatase, alkaline (ALP)
BACKGROUND
Alkaline phosphatases are enzymes that originate in multiple tissues/organs including the liver, bone, kidney and placenta. During pregnancy, ALP levels physiologically increase as pregnancy progresses primarily as a result of placental and bone isoenzymes. Isolated increases in ALP are not diagnostic and typically do not warrant further investigation. Total ALP values may be significantly increased with certain pregnancy-related conditions such as preeclampsia, cholestasis, intervillositis, SGA and when the pregnant person smokes during pregnancy. However, the clinical significance and diagnostic value of elevated ALP is unclear.
COMMON INDICATIONS
- Investigations for liver or bone disease/conditions
- NB: The SOGC does not include ALP in it’s recommended lab investigations for HDP or ICP
SAMPLE COLLECTION
- Serum sample in a gold, red or green vacutainer
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| U/L | 33 - 96 | 17 - 88 | 25 - 126 | 38 - 229 |
| µkat/L | 0.55 - 1.6 | 0.28 - 1.47 | 0.43 - 2.1 | 0.63 - 3.82 |
SOURCES
- Guarino M, Cossiga V, Morisco F. The interpretation of liver function tests in pregnancy. Best practice & research Clinical gastroenterology. 2020 Feb 1;44:101667.
- Titaux C, Ternynck C, Pauchet M, Stichelbout M, Bizet G, Maboudou P, Onraed B, Clément G, Lenne X, Potier G, Subtil D. Total alkaline phosphatase levels by gestational age in a large sample of pregnant women. Placenta. 2023 Feb 1;132:32-7.
- https://www.perinatology.com/Reference/Reference%20Ranges/Alkaline%20phosphatase.htm
Placental growth factor (PlGF)
BACKGROUND
Placental growth factor (PlGF) is a pro-angiogenic growth factor produced and released by placental villi into the circulatory system of the pregnant person. Low PlGF is thought to reflect syncytiotrophoblast stress and poor placentation.
PlGF is a serum biomarker increasingly supported by evidence as a diagnostic test for timely identification of preeclampsia. Low levels of circulating PlGF is linked to increased risk of preeclampsia, particularly preterm or early onset preeclampsia.
COMMON INDICATIONS
- Included in multiple marker prenatal screening for preeclampsia
- Included in eFTS screening at some provincial labs in Ontario (North York General and Trillium Credit Valley)
SAMPLE COLLECTION
- Serum sample in serum separator tube (preferred) - red or gold vacutainer tube
reference values
- Reference values will vary by gestational age
- Retrospective analyses with data from Mount Sinai Hospital Toronto considered a value <100 pg/mL as low PlGF in multiple marker screening for preeclampsia (McLaughlin 2021)
- In research related to PlGF in preeclampsia screening, the value has been identified as low PlGF when <5th %ile for PlGF results within a gestational age range (<35, 35-36+6, ≥37) (Chappell 2013)
SOURCES
For more information, watch this AOM webinar: Prenatal screening for preeclampsia: The Canadian experience (Jan 2022)
- AOM. Clinical practice guideline 15: Hypertensive disorders of pregnancy. 2023. Available from: https://www.ontariomidwives.ca/cpg
- Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, Simpson N, Waugh J, Anumba D, Kenny LC, Redman CW. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013 Nov 5;128(19):2121-31.
- McLaughlin K, Audette MC, Banner H, Barrett J, Bujold E, Chen H, Cohen N, Delaney T, Figueiro E, Gladstone RA, Jain V. Placental Growth Factor (PlGF) diagnostic testing: An opportunity to transform pregnancy care for patients with suspected preeclampsia in Canada. Journal of Obstetrics and Gynaecology Canada. 2025 Aug 14:103076.
- McLaughlin K, Snelgrove JW, Audette MC, et al. PlGF (Placental Growth Factor) testing in clinical practice: evidence from a Canadian Tertiary Maternity Referral Center. Hypertension 2021;77:2057—65.
Polymerase Chain Reaction (PCR), urine testing and serology for Zika virus
BACKGROUND
Zika virus (ZIKV) infection is caused by a flavivirus transmitted through the bite of an infected Aedes mosquito. ZIKV RNA has been detected in semen and sexual transmission has been confirmed. Knowledge of the virus and cases of human infection have been documented since the 1950s. Starting in the early 2000s and peaking in 2016, ZIKV caused major outbreaks in many tropical and subtropical areas of the world, especially in the Americas. During this time, adverse pregnancy outcomes (such as congenital microcephaly) were identified related to ZIKV infection during pregnancy. These adverse outcomes are collectively referred to as a congenital Zika syndrome (CZS).
- From PHAC: “There has been a substantial reduction in the risk of Zika virus (ZIKV) infection among Canadian travellers. Accordingly, CATMAT no longer routinely recommends that pregnant travellers avoid travel to areas where Zika is known or suspected to occur, or that special precautions to prevent sexual transmission while abroad or upon return are necessary.”
Serologic testing for Zika has become increasingly problematic for several reasons. Specificity has always been relatively low due to cross reactivity with other related flaviviruses, and positive predictive value has fallen as prevalence has decreased in recent years. At the start of the epidemic, the presence of antibodies suggested recent exposure. At the present time, however, seropositivity may often represent remote exposure. In addition, data shows that IgM also may remain positive for over 2 years, thus limiting specificity for recent infection
COMMON INDICATIONS
Asymptomatic
-
Given the low risk of ZIKV infection, CATMAT recommends against routine testing of asymptomatic pregnant people. Exceptions could be considered when the risk of exposure is particularly high, and the psychological benefit of a negative result clearly outweighs the harms which could arise from a false positive result.
Symptomatic
-
Testing should be offered to pregnant people with acute signs and symptoms compatible with ZIKV.
SAMPLE COLLECTION
- For postnatal diagnosis of congenital infection, PCR for ZIKV can be performed on placental tissue, umbilical cord blood or infant blood. Collect within 2 days of birth.
- NB: It is possible that infants or fetuses infected weeks prior to specimen sampling will no longer have detectable viral RNA.
- For pregnant people, RT-PCR on blood and urine is the preferred testing modality.
- Serum sample collected in serum gel tube (gold) or red vacutainer. Collect 2 full tubes for ZIKV PCR testing.
- Urine samples collected in sterile urine container using "regular catch" technique (not midstream), min 5 mL.
- ZIKV PCR can be performed on serum and urine collected up to 12 weeks after symptom onset
SOURCES
- Public Health Ontario: https://www.publichealthontario.ca/en/Laboratory-Services/Test-Information-Index/Zika-Virus
- PHAC: https://www.canada.ca/en/public-health/services/diseases/zika-virus.html
Potassium
BACKGROUND
Potassium is an essential mineral and the main cation in intracellular fluid of all cells in the human body. Potassium is required for normal cell function because of its role in maintaining intracellular fluid volume and transmembrane electrochemical gradients.
- Hypokalemia is one of the most common fluid-and-electrolyte abnormalities encountered in clinical practice, and occurs in < 1% of all pregnancies. Hyperemesis gravidarum (HG) is strongly associated with hypokalemia. One study found about 20% of pregnancy-related hospitalizations with hypokalemia also had a diagnosis of HG, while HG occurred in less than 1% of patients without hypokalemia. Additional risk factors for perinatal hypokalemia include infection, hypertensive disorders and postpartum hemorrhage.
- Hyperkalemia is rare in the peripartum period. Conditions such as chronic kidney disease may increase the risk of hyperkalemia. Case studies have found associations with perinatal hyperkalemia and use of certain medications used to treat gestational hypertension/preeclampsia (magnesium sulfate, labetolol), intrauterine fetal demise, and refeeding syndrome following treatment for hyperemesis gravidarum – though these are all rare occurrences.
COMMON INDICATIONS
- Refractory NVP, hyperemesis gravidarum monitoring
SAMPLE COLLECTION
- Serum sample in green, gold or red top vacutainer tube
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| mEq/L | 3.5 - 5 | 3.6 - 5 | 3.3 - 5 | 3.3 - 5.1 |
| mmol/L | 3.5 - 5 | 3.6 - 5 | 3.3 - 5 |
3.3 - 5.1 |
SOURCES
-
He J, Morton A. Hypokalaemia in pregnancy–Prevalence, underlying causes, and an approach to investigation. Obstetric Medicine. 2024 Dec;17(4):213-20.
-
Sur M, Mohiuddin SS. Potassium. InStatPearls [Internet] 2024 Oct 5. StatPearls Publishing.
-
Yang CW, Li S, Dong Y. The Prevalence and Risk Factors of Hypokalemia in Pregnancy‐Related Hospitalizations: A Nationwide Population Study. International Journal of Nephrology. 2021;2021(1):9922245.
- https://www.perinatology.com/Reference/Reference%20Ranges/Potassium.htm
Protein, total
BACKGROUND
- Plasma proteins, namely albumin and globulin, have a role in maintaining colloidal osmotic (oncotic) pressure. Albumin comprises 50–60% of the total protein content of blood. Because plasma proteins are relatively large, they do not easily cross capillary walls, which counteracts fluid loss from capillaries into tissues by creating an osmotic gradient. When plasma proteins are reduced, for example in conditions involving proteinuria, the result is a reduction in the osmotic pressure resulting in fluid buildup in the tissues (edema).
- The concentration of blood biomarkers in pregnant people is influenced by plasma volume expansion, and causes of hypoproteinemia in pregnancy can be very varied, including hemodilution, increased kidney clearance, and higher usage of proteins on behalf of the fetus and the pregnant person’s organs.
COMMON INDICATIONS
- Not routinely ordered in pregnancy, but may be ordered for certain investigations related to liver, kidney or dietary concerns.
SAMPLE COLLECTION
- Blood test, red or gold top vacutainer
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| g/dL | 6.7 - 8.6 | 6.2 - 7.6 | 5.7 - 6.9 | 5.6 - 6.7 |
| g/L | 67 - 86 | 62 - 76 | 57 - 69 | 56 - 67 |
SOURCES
- https://www.perinatology.com/Reference/Reference%20Ranges/Protein.htm
Protein, total (24-hour urine, total protein)
BACKGROUND
- A 24-hour urine collection for total protein is the gold standard diagnostic test for proteinuria (though other testing may be implemented when a rapid timing of diagnosis is important).
- Outside of pregnancy, urine contains very little protein with health adults excreting <150 mg of protein per day in urine. In a healthy pregnancy, there is approximately double (up to 260 mg/day) the amount of protein in urine due to physiologic adaptations of pregnancy. Pathologic proteinuria is typically defined with a cutoff of > 300 mg/24 h. Conditions in pregnancy like preeclampsia impact kidney function, resulting in protein ‘leaking’ into the urine.
COMMON INDICATIONS
- Testing for HDP: suspected preeclampsia and diagnosis of proteinuria (e.g., follow up for positive dipstick for proteinuria)
- Investigation of secondary causes of hypertension (e.g., primary aldosteronism)
SAMPLE COLLECTION
- For 24-hour urine samples, it is important that the correct collection procedure is observed. Provide your client with information specific to your laboratory/community/institution.
- Some examples of client handouts for 24-hour urine collection: The Ottawa Hospital, Humber River Hospital, Mount Sinai Hospital, and Thunder Bay RHSC.
- See also: Lifelabs provider overview page.
reference values
- Proteinuria is defined as ≥ 0.3 g/day (≥ 300 mg/day) in a 24h urine sample.
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| mg/24h | < 150 | - | 0 - 255 | 0 - 254 |
| g/24h | < 0.15 | - | 0 - 0.26 | 0 - 0.25 |
SOURCES
- AOM. Clinical practice guideline 15: Hypertensive disorders of pregnancy. 2023. Available from: https://www.ontariomidwives.ca/cpg
- Bartal MF, Lindheimer MD, Sibai BM. Proteinuria during pregnancy: definition, pathophysiology, methodology, and clinical significance. American journal of obstetrics and gynecology. 2022 Feb 1;226(2):S819-34.
- https://www.perinatology.com/Reference/Reference%20Ranges/Protein%20excretion.htm
T-3, total (triiodothyronine)
BACKGROUND
Triiodothyronine (T-3) is a thyroid hormone produced by the thyroid gland and peripherally through deiodination of T4. Total T-3 is a blood test measuring both bound (inactive) and free (active) T3 and is used in the investigation of thyroid conditions such as hyperthyroidism. T3 has a higher affinity for thyroid hormone receptors than T4 and so exerts the majority of thyroid hormone activity, even thought its circulating levels are lower than T4. T3 levels (especially free T3) may guide evaluation of thyroid dysfunction but are less commonly used alone due to variability and peripheral conversion dynamics.
COMMON INDICATIONS
Routine screening of Total T3 for asymptomatic clients is not recommended. T3 may be tested as part of a more in-depth thyroid assessment or when a known thyroid condition is pre-existing to assess severity and/or guide management.
SAMPLE COLLECTION
Serum sample, gold top or red top vacutainer (some labs may also accept green top tubes).
reference values
- Typically total T3 will increase as pregnancy advances in gestation.
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| ng/dL | 77 - 135 | 97 - 149 | 117 - 169 | 123 - 162 |
| nmol/L | 1.19 - 2.08 | 1.49 - 2.29 | 1.8 - 2.6 | 1.89 - 2.29 |
SOURCES
- https://www.perinatology.com/Reference/Reference%20Ranges/Triiodothyronine,total.htm
-
Almomin AM, Mansour AA, Sharief M. Trimester-specific reference intervals of thyroid function testing in pregnant women from Basrah, Iraq using electrochemiluminescent immunoassay. Diseases. 2016 Apr 26;4(2):20.
-
Brett AS. Reducing Unnecessary Ordering of T3 Tests. NEJM Journal Watch. 2022:NA55605.
T-4, free absolute
BACKGROUND
Thyroxine (T4) is the primary hormone produced by the thyroid gland. A Free T4 test measures the amount of free (unbound) biologically active thyroxine hormone in the blood. It does not measure the bound T4. T4 testing is used in the evaluation of thyroid function and the diagnosis hypothyroidism or hyperthyroidism, often in conjunction with thyroid-stimulating hormone (TSH) testing or as a follow up to abnormal TSH.
COMMON INDICATIONS
Routine screening in asymptomatic pregnant persons is not recommended. Consider as a follow up to an abnormal TSH, or in conjunction with TSH when suspecting thyroid condition. May help guide management for known thyroid conditions to ensure appropriate dosing.
SAMPLE COLLECTION
Serum sample in a red, gold or green vacutainer.
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| ng/dL | 0.8 - 1.7 | 0.8 - 1.2 | 0.6 - 1 | 0.5 - 0.8 |
| pmol/L | 10.3 - 21.9 | 10.3 - 15.5 | 7.7 - 12.9 | 6.4 - 10.3 |
Interpretation guide for hypothyroidism (general)
TSH |
T4 |
Dx |
| High (≥ 10 mIU/L) | Low | Primary hypothyroidism |
| Normal or low | Low | Central or secondary hypothyroidism |
|
Slightly high (but ≤ 9.99 mIU/L) |
Normal | Subclinical hypothyroidism |
SOURCES
- https://www.perinatology.com/Reference/Reference%20Ranges/Thyroxine,%20free.htm
- Soldin OP. Thyroid function testing in pregnancy and thyroid disease: trimester-specific reference intervals. Therapeutic drug monitoring. 2006 Feb 1;28(1):8-11.
TSH (thyroid stimulating hormone)
BACKGROUND
Thyroid-stimulating hormone (TSH) is a pituitary hormone that regulates thyroid gland activity. It responds to circulating free T4 and T3 levels through a negative feedback system.
COMMON INDICATIONS
- TSH is the primary screening and monitoring test for thyroid dysfunction in pregnancy.
- Routine screening for low-risk asymptomatic clients is not recommended. Consider screening for those with significant history or risk factors, or who are symptomatic. Regular TSH monitoring for those with diagnosed thyroid condition can guide management, including medication dosing.
- There is insufficient evidence to recommend routine screening for postpartum thyroiditis. Postpartum thyroiditis is an autoimmune condition that occurs in approximately 5% of people in the first year postpartum; it is often mild and transient.
- TSH is a routine component of Newborn Screen Ontario blood spot test, screening for congenital hypothyroidism (CH).
SAMPLE COLLECTION
Adult sampling: blood test collected in a gold, red or green tube.
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| µIU/mL or mIU/L | 0.34 - 4.25 | 0.6 - 3.4 | 0.37 - 3.6 | 0.38 - 4.04 |
Interpretation guide for hypothyroidism in adults (general - consider full clinical picture and consult with specialists as indicated)
TSH |
T4 |
Dx |
| High (≥ 10 mIU/L) | Low | Primary hypothyroidism |
| Normal or low | Low | Central or secondary hypothyroidism |
|
Slightly high (but ≤ 9.99 mIU/L) |
Normal | Subclinical hypothyroidism |
SOURCES
- Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstetrics & Gynecology. 2009 Dec 1;114(6):1326-31.
- Soldin OP. Thyroid function testing in pregnancy and thyroid disease: trimester-specific reference intervals. Therapeutic drug monitoring. 2006 Feb 1;28(1):8-11.
- Yamamoto JM, Metcalfe A, Nerenberg KA, Khurana R, Chin A, Donovan LE. Thyroid function testing and management during and after pregnancy among women without thyroid disease before pregnancy. CMAJ. 2020 Jun 1;192(22):E596-602.
- BC Guidelines. Thyroid Function Testing in the Diagnosis and Monitoring of Thyroid Function Disorder. 2018 Oct 24. Online.
Uric acid (Urate)
BACKGROUND
Uric acid is a byproduct of purine metabolism and is associated with endothelial dysfunction and oxidative stress characteristic of preeclampsia. It is a marker of renal dysfunction and tissue injury. Elevated serum uric acid is associated with increased perinatal risk and placental dysfunction, but the clinical utility of this finding in practice is unclear in the literature.
COMMON INDICATIONS
Not recommended as routine part of HDP screening by SOGC (2022), but if performed may help guide management around determining need for additional fetal monitoring.
SAMPLE COLLECTION
Blood sample in a red top or gold top vacutainer.
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| mg/dL | 2.5 - 5.6 | 2 - 4.2 | 2.4 - 4.9 | 3.1 - 6.3 |
| µmol/L | 149 - 333 | 119 - 250 | 143 - 292 | 184 - 375 |
SOURCES
- https://www.perinatology.com/Reference/Reference%20Ranges/Uric%20acid.htm
-
Deeksha HS, Pajai S, Eleti MR, Navalihiremath VU. A comprehensive review on serum Lactate Dehydrogenase (LDH) and uric acid in preeclampsia: implications for maternal health and disease severity. Cureus. 2024 Mar 25;16(3).
-
Magee LA, Smith GN, Bloch C, Côté AM, Jain V, Nerenberg K, von Dadelszen P, Helewa M, Rey E. Guideline No. 426: hypertensive disorders of pregnancy: diagnosis, prediction, prevention, and management. Journal of Obstetrics and Gynaecology Canada. 2022 May 1;44(5):547-71.
-
Pecoraro V, Trenti T. Predictive value of serum uric acid levels for adverse maternal and perinatal outcomes in pregnant women with high blood pressure. A systematic review and meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2020 Sep 1;252:447-54.
Varicella zoster virus (VZV)
BACKGROUND
Varicella, or chickenpox, is a common highly infectious viral disease caused by the varicella-zoster virus (VZV), a DNA virus of the herpesvirus family. The defining symptom is a blister-like rash involving skin lesions on all areas of the body which can cause severe irritation. Following the initial varicella illness, varicella-zoster virus establishes latency in the sensory nerve ganglia, which may be reactivated later in life as herpes zoster (also known as shingles). It may take 10 to 21 days for symptoms to appear after infection has occurred. A person is most contagious from 1 to 2 days before to shortly after the onset of rash. Contagiousness persists until the skin lesions crust over. The National Advisory Committee on Immunization (NACI) recommends immunization against varicella (NB: Varicella vaccination is contraindicated during pregnancy, consider varicella zoster immune globulin administration in pregnancy as indicated).
Congenital varicella syndrome is rare when infection occurs before the 13th or after the 20th week of gestation. The risk is approximately 2% when infection occurs at 13-19 weeks of gestation. Congenital infection results in a wide clinical spectrum, which may include low birth weight, ophthalmic abnormalities, skin scarring, limb atrophy, cerebral atrophy and a variety of other anomalies. Almost one-third of affected infants die by early in the second year of life. Varicella infection for the pregnant person occurring in the 5 days before to 2 days after birth is associated with severe neonatal varicella in 17% to 30% of infants, with high case fatality for the newborn. (PHAC 2025)
COMMON INDICATIONS
Varicella serology testing should be considered if:
- Pregnant and no past history of varicella, or exposure to varicella during pregnancy and immunity status was based on history rather than serology results.
- Pregnant and no clear past history of exposure/vaccination (NB: vaccination has low availability in many countries globally)
SAMPLE COLLECTION
- For immunity and/or diagnostic serology: blood test in a serum separator tube (red top vacutainer). Processed by Public Health Ontario.
SOURCES
- Shrim A, Koren G, Yudin MH, Farine D. No. 274-Management of Varicella infection (chickenpox) in pregnancy. Journal of Obstetrics and Gynaecology Canada. 2018 Aug 1;40(8):e652-7.
- Public Health Ontario. Test information: Varicella serology. https://www.publichealthontario.ca/en/Laboratory-Services/Test-Information-Index/Varicella-Serology
- Public Health Canada: https://www.canada.ca/en/public-health/services/immunization/vaccine-preventable-diseases/varicella-chickenpox.html
Vitamin B12
BACKGROUND
Vitamin B12 (cobalamin) is a complex, water-soluble vitamin found in food derived from animal products and in fortified foods, and is required for proper red blood cell formation, cell metabolism, neurological function and DNA synthesis.
A deficiency in vitamin B12 is associated with a number of issues including macrocytic (megaloblastic) anemia and neurologic sequelae, hyperglycemia, insulin resistance, obesity, and fetal neural tube defects (vitamin B12 has a key role in intrauterine fetal development). Supplementation with B12 should be considered by all people during pregnancy and during chest/breastfeeding, especially those with a vegan or vegetarian diet or with minimal intake of animal-derived foods (meat, dairy, eggs, etc.).
COMMON INDICATIONS
- Routine screening in asymptomatic persons is not recommended. For those with risk factors for B12 deficiency, consider supplementation in lieu of screening test.
- Ruling out B12 deficiency as cause of macrocytic anemia.
SAMPLE COLLECTION
- Blood sample collected in gold top vacutainer
reference values
- NB: taking oral contraceptives may result in decreased serum B12 levels in the absence of clinical deficiency (due to decreases in the B12 carrier protein, haptocorrin).
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| pg/mL | 279 - 966 | 118 - 438 | 130 - 656 | 99 - 526 |
| pmol/L | 206 - 713 | 87 - 323 | 96 - 484 | 73 - 388 |
SOURCES
- https://www.perinatology.com/Reference/Reference%20Ranges/Vitamin%20B12.htm
- BC Guidelines. Cobalamin (vitamin B12) and Folate Deficiency. 2023 January 18. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12
- Cruz-Rodríguez J, Díaz-López A, Canals-Sans J, Arija V. Maternal vitamin B12 status during pregnancy and early infant neurodevelopment: The ECLIPSES study. Nutrients. 2023 Mar 22;15(6):1529.
- Al Amin AS, Gupta V. Vitamin B12 (cobalamin). July 2023. Online : https://www.ncbi.nlm.nih.gov/books/NBK559132/
- Wilson RD, Van Mieghem T, Langlois S, Church P. Guideline No. 410: Prevention, screening, diagnosis, and pregnancy management for fetal neural tube defects. Journal of Obstetrics and Gynaecology Canada. 2021 Jan 1;43(1):124-39.
Vitamin D, 25-hydroxy
BACKGROUND
Vitamin D is fat soluble and stored in body fat. It is produced in the skin during sun exposure, and very few foods are a natural source of vitamin D though some foods may be fortified (e.g., orange juice, milk). Adequate vitamin D is required to maintain the serum calcium concentration within the normal physiologic range for musculoskeletal health. Pregnant people are at higher risk for vitamin D deficiency, and can consider routine supplementation throughout pregnancy and chest/breastfeeding.
COMMON INDICATIONS
- Routine screening in pregnancy is not recommended, and is of low clinical utility for those suspected of being at risk and who would benefit from supplementation.
- Vitamin D testing is indicated in patients who are at high risk for vitamin D deficiency such as those with malabsorption syndromes, renal failure, unexplained bone pain, unusual fractures, or other evidence of metabolic bone disorders.
- 25-hydroxyvitamin D is the preferred test for assessing vitamin D status.
SAMPLE COLLECTION
- Blood sample collected in a gold-top vacutainer tube.
reference values
| Units | Nonpregnant adult | 1st trimester | 2nd trimester | 3rd trimester |
|---|---|---|---|---|
| ng/mL | 14 - 80 | 18 - 27 | 10 - 22 | 10 - 18 |
| nmol/L | 35 - 200 | 45 - 67 | 25 - 55 | 25 - 45 |
SOURCES
- https://www.perinatology.com/Reference/Reference%20Ranges/Vitamin%20D,25%20hydroxy.htm
Point of care tests (POCTs)
Point of care test |
Common indications |
Modalities of testing (examples, not exhaustive) |
| Amniotic swab | PPROM/PROM assessment, confirming ROM | Nitrazine swab (pH testing), swab and ferning test, AmniSure (PAMG-1 protein test) |
| Glucose | Neonatal hypoglycemia monitoring, GDM | Glucometer (capillary blood glucose) |
| Pregnancy test | Pregnancy confirmation, monitoring following loss/abortion | Urine hCG |
| Strep B rapid screen | Unknown GBS screening result at the time of labour onset | Vaginal-rectal swab (PCR assay test, e.g., “Xpert”) |
| Urine dipstick | Elevated blood pressure and/or s/sx of preeclampsia (proteinuria test) | Urine test strips; either manual interpretation (with colour chart) or automated (machine conducts reflectance photometry) |

